SPOTO ASQ Certification Dumps List
-
CATEGORIESEXAM TRACKSEXAM QUESTION NUMBERDUMP COVERAGEPass Exam DUMPSFREE TEST
-
ASQ500+100% cover
-
ASQ500+100% cover
-
ASQ500+100% cover
-
ASQ500+100% cover
-
ASQ500+100% cover
-
ASQ500+100% cover
-
ASQ500+100% cover
-
ASQ500+100% cover
-
ASQ500+100% cover
-
ASQ500+100% cover
-
ASQ500+100% cover
-
ASQ500+100% cover
-
ASQ500+100% cover
-
ASQ500+100% cover
-
Luc***2026/03/07order Other ***Hen***2026/03/07order Other ***Ale***2026/03/07order Other ***
-
Mas***2026/03/07order Other ***Mic***2026/03/07order Other ***Eth***2026/03/07order Other ***
-
Dan***2026/03/07order Other ***Jac***2026/03/07order Other ***Log***2026/03/07order Other ***
-
Jac***2026/03/07order Other ***Lev***2026/03/07order Other ***Seb***2026/03/07order Other ***
-
Mat***2026/03/07order Other ***Jac***2026/03/07order Other ***Owe***2026/03/07order Other ***
-
The***2026/03/07order Other ***Aid***2026/03/07order Other ***Sam***2026/03/07order Other ***
-
Lia***2026/03/07order Other ***Noa***2026/03/07order Other ***Oli***2026/03/07order Other ***
-
Eli***2026/03/07order Other ***Wil***2026/03/07order Other ***Jam***2026/03/07order Other ***
-
Luc***2026/03/07order Other ***
-
Mas***2026/03/07order Other ***
-
Dan***2026/03/07order Other ***
-
Jac***2026/03/07order Other ***
-
Owe***2026/03/07order Other ***
-
The***2026/03/07order Other ***
-
Lia***2026/03/07order Other ***
-
Wil***2026/03/07order Other ***
Medical Device Auditor CMDA Exam Overview
The ASQ Certified Medical Device Auditor credential demonstrates your ability to audit medical-device quality systems against international standards and regulatory requirements. You’ll apply tools and techniques to examine, question, and evaluate system adequacy—identifying gaps and recommending corrective actions.

ASQ Certified Medical Device Auditor
CMDA
145 multiple-choice questions
270 minutes
550 out of 750
$533
Experience Requirements:
5 years of on-the-job experience in one or more CMDA Body of Knowledge areas. Minimum 1 year in a decision-making roleThe ASQ Certified Medical Device Auditor credential demonstrates your ability to audit medical-device quality systems against international standards and regulatory requirements. You’ll apply tools and techniques to examine, question, and evaluate system adequacy—identifying gaps and recommending corrective actions.

ASQ Certified Medical Device Auditor
CMDA
145 multiple-choice questions
270 minutes
550 out of 750
$533
Experience Requirements:
5 years of on-the-job experience in one or more CMDA Body of Knowledge areas. Minimum 1 year in a decision-making roleASQ CMDA Exam Topics & SPOTO Question Bank
1.CMDA Exam Syllabus Topics
I. Auditing Fundamentals (12 Questions)
A. Types of Audits
B. Audit Roles and Responsibilities
C. Ethical, Legal, and Professional Issues
II. Auditing and Inspection Processes (28 Questions)
A. Audit Preparation and Planning
B. Audit Performance
C. Audit Report
D. Audit Follow-Up and Closure
E. Audit Procedural References
III. Medical Device Quality Management System Requirements (38 Questions)
A. Regulatory Laws and Requirements
B. In Vitro Diagnostic (IVD) Devices
C. International Quality System Standards
D. Quality System Regulation (QSR)
E. Post-Market Surveillance
IV. Technical Medical Device Knowledge (42 Questions)
A. Risk Management
B. Design Control
C. Software Development & Maintenance
D. Labeling Requirements
E. Controlled Environments & Utilities
F. Sterile Device Processes
G. Laboratory Testing & Failure Analysis
V. Quality Tools and Techniques (15 Questions)
A. Quality Control & Problem-Solving Tools
B. Process Improvement Techniques
C. Data Types & Sampling Methods
2.What Is the ASQ CMDA Question Dumps?
SPOTO's CMDA dumps feature a rich collection of real-world audit scenarios and multiple-choice questions that span every domain of the Body of Knowledge. Authored and peer-reviewed by seasoned medical-device auditors, our bank mimics the format and complexity of the live 145-question exam—ensuring you gain hands-on familiarity with audit checklists, reporting formats, and regulatory references. With over twice as many practice items as the actual exam, you'll reinforce your understanding of audit fundamentals, technical device standards, and quality-system requirements in just 7-10 days.
3.Why Choose ASQ CMDA Exam Questions and Answers?
SPOTO delivers the most accurate, up-to-date CMDA exam materials on the market. Our full-length mock exams mirror the 270-minute, open-book format, while every answer is accompanied by detailed rationales grounded in FDA, ISO 13485, and global regulatory guidelines. Personalized tutoring sessions help you zero in on your weakest areas—whether it's sterile-processing audits or post-market surveillance—so you walk into the exam room fully prepared.
4.ASQ CMDA Application Process & Timeline
-
Submit Your Application
-
Online submissions are reviewed within 1-2 business days.
-
Paper applications take 3-5 business days for initial review.
-
-
ASQ Evaluation
-
Once ASQ completes its review, you'll receive an approval email or a “hold” notice requesting additional information.
-
If you don't meet the eligibility criteria, ASQ will refund your fee minus a $130 processing charge.
-
-
Testing Formats
-
Computer-Based Testing (CBT):
Within 1-2 business days of approval, you'll get an eligibility email with scheduling instructions for Prometric. -
Paper-Based Testing (PBT):
For paper-based or translated exams, you won't receive an eligibility email. Instead, you'll get a seating letter—detailing your exam date, time, and location—about two weeks before the test.
-
-
Schedule Your Exam
Book your appointment with Prometric during the testing window you selected when applying.
Latest Passing Reports from SPOTO Candidates
RELATED Practice test
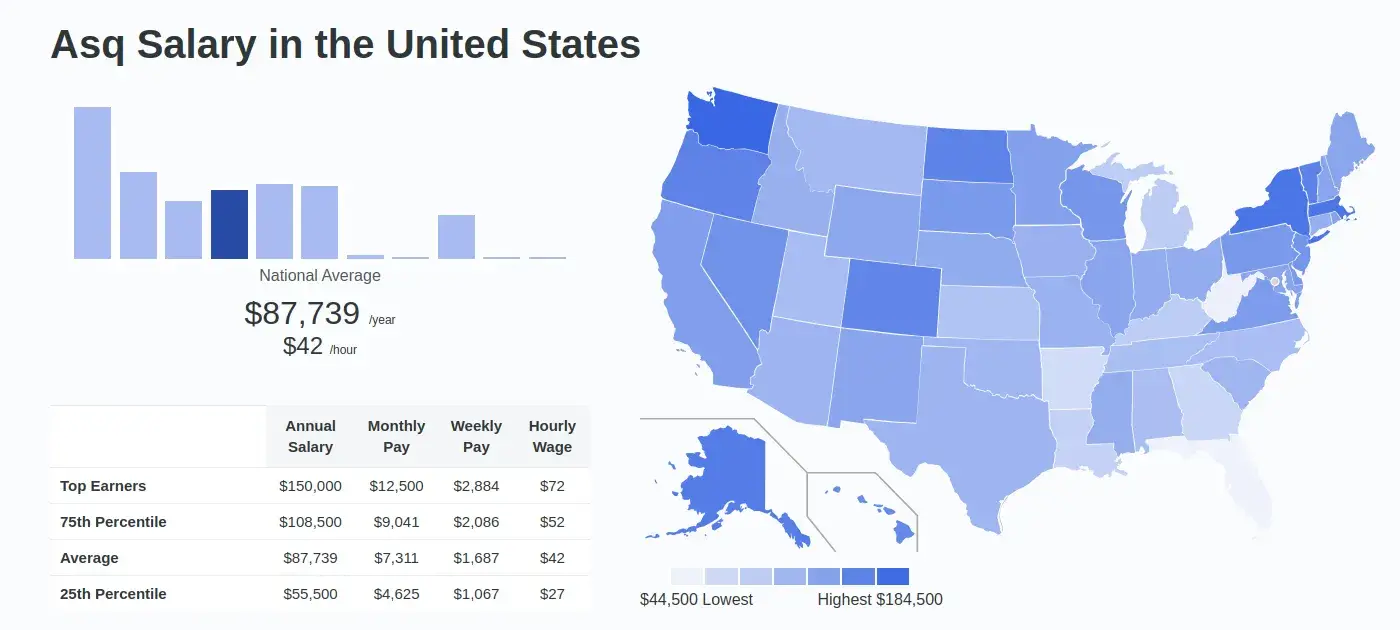
How Much Can You Make with the Certification?
Certified professionals can earn up to 40-percent more than their non-certified peers.
ASQ CMDA Exam Dumps FAQs
1.Are the exam screenshots provided by SPOTO real?
Absolutely. Every screenshot in our CMDA package comes from authentic candidate experiences, reflecting the same question formats, audit checklists, and regulatory excerpts you'll see on exam day.
2.Can I avail special discounts?
Yes. SPOTO offers seasonal promotions, bulk-purchase deals, and loyalty discounts. Contact our sales team to learn about current offers and package pricing.
3.How long is the service period of the CMDA dumps?
Your initial access lasts 7-10 days ample time to complete multiple full-length mock exams and targeted drills. Extensions and renewals are available if you need more preparation time.
4.How many questions are there in the CMDA exam dumps?
To ensure thorough coverage, SPOTO provides 2-3× more practice questions than the actual 145-item exam. This extra breadth guarantees you've rehearsed every audit scenario before test day.
5.What support does SPOTO offer during my study period?
Enjoy 24-hour access to our expert tutors via chat, plus topic-focused quizzes and performance analytics to track your progress and pinpoint areas for improvement.