SPOTO ASQ Certification Dumps List
-
CATEGORIESEXAM TRACKSEXAM QUESTION NUMBERDUMP COVERAGEPass Exam DUMPSFREE TEST
-
ASQ500+100% cover
-
ASQ500+100% cover
-
ASQ500+100% cover
-
ASQ500+100% cover
-
ASQ500+100% cover
-
ASQ500+100% cover
-
ASQ500+100% cover
-
ASQ500+100% cover
-
ASQ500+100% cover
-
ASQ500+100% cover
-
ASQ500+100% cover
-
ASQ500+100% cover
-
ASQ500+100% cover
-
ASQ500+100% cover
-
Luc***2026/03/07order Other ***Hen***2026/03/07order Other ***Ale***2026/03/07order Other ***
-
Mas***2026/03/07order Other ***Mic***2026/03/07order Other ***Eth***2026/03/07order Other ***
-
Dan***2026/03/07order Other ***Jac***2026/03/07order Other ***Log***2026/03/07order Other ***
-
Jac***2026/03/07order Other ***Lev***2026/03/07order Other ***Seb***2026/03/07order Other ***
-
Mat***2026/03/07order Other ***Jac***2026/03/07order Other ***Owe***2026/03/07order Other ***
-
The***2026/03/07order Other ***Aid***2026/03/07order Other ***Sam***2026/03/07order Other ***
-
Lia***2026/03/07order Other ***Noa***2026/03/07order Other ***Oli***2026/03/07order Other ***
-
Eli***2026/03/07order Other ***Wil***2026/03/07order Other ***Jam***2026/03/07order Other ***
-
Luc***2026/03/07order Other ***
-
Mas***2026/03/07order Other ***
-
Dan***2026/03/07order Other ***
-
Jac***2026/03/07order Other ***
-
Owe***2026/03/07order Other ***
-
The***2026/03/07order Other ***
-
Lia***2026/03/07order Other ***
-
Wil***2026/03/07order Other ***
Certified Pharmaceutical GMP Professional Exam Overview
A Certified Pharmaceutical GMP Professional possesses in‐depth expertise in the regulatory frameworks and guidelines that govern good manufacturing practices for the pharmaceutical sector. This includes oversight of finished human and veterinary medications, biologics, ectoparasiticides, and nutraceuticals treated as drug products, as well as their constituent raw materials (such as active pharmaceutical ingredients and excipients), plus all associated packaging and labeling processes.

Certified Pharmaceutical GMP Professional
ASQ CPGP
165 multiple-choice questions
Multiple-choice
4 hours 18 minutes
English
Format:
Optional computer-based or paper-based; all exams are open-bookA Certified Pharmaceutical GMP Professional possesses in‐depth expertise in the regulatory frameworks and guidelines that govern good manufacturing practices for the pharmaceutical sector. This includes oversight of finished human and veterinary medications, biologics, ectoparasiticides, and nutraceuticals treated as drug products, as well as their constituent raw materials (such as active pharmaceutical ingredients and excipients), plus all associated packaging and labeling processes.

Certified Pharmaceutical GMP Professional
ASQ CPGP
165 multiple-choice questions
Multiple-choice
4 hours 18 minutes
English
Format:
Optional computer-based or paper-based; all exams are open-bookCertified Pharmaceutical GMP Professional Exam Topics & SPOTO Question Bank
1.Body of Knowledge Topics:
-
Regulatory Agency Governance
-
Quality Systems
-
Laboratory Systems
-
Infrastructure: Facilities, Utilities, Equipment
-
Materials and Supply Chain Management
-
Sterile and Nonsterile Manufacturing Systems
-
Filling, Packaging, and Labeling
-
Product Development and Technology Transfer
2.Exam Requirements:
-
5 years of relevant, on-the-job GMP experience
-
Minimum 3 years in a decision-making capacity
3.Which GMP regulations will the exam primarily focus on?
-
United States FDA GMPs
-
European Union (EU) GMPs / PIC/S / TGA
-
World Health Organization (WHO) GMPs
-
Japanese GMPs
-
Canadian GMPs
4.What is the ASQ CPGP Exam Question Bank?
SPOTO's ASQ CPGP dumps deliver a carefully curated collection of authentic exam questions and answers—from definitions of global GMP regulations to scenario-based quality system challenges. Every question is cross-verified by experienced GMP professionals, mirroring the format, difficulty, and breadth of the live 165-question exam. With over twice as many practice items as the actual test, you'll reinforce your understanding of APIs, excipients, facility design, and more—so you can work through each topic in depth and build unshakable confidence.
5.Why choose Certified Pharmaceutical GMP Professional Exam Questions and Answers?
Our ASQ CPGP question bank is the industry's most current and comprehensive resource, reflecting updates across FDA, EU-PIC/S, WHO, TGA, Japanese, and Canadian GMPs. SPOTO's full-length mock exams replicate the open-book format and time constraints of your 4 hour 18 minute testing session, while detailed rationales ensure you grasp both the correct answers and their regulatory underpinnings. Complementary 1-on-1 tutoring and targeted quizzes let you focus on your weakest domains—so you're thoroughly prepared on exam day.
Latest Passing Reports from SPOTO Candidates
RELATED Practice test
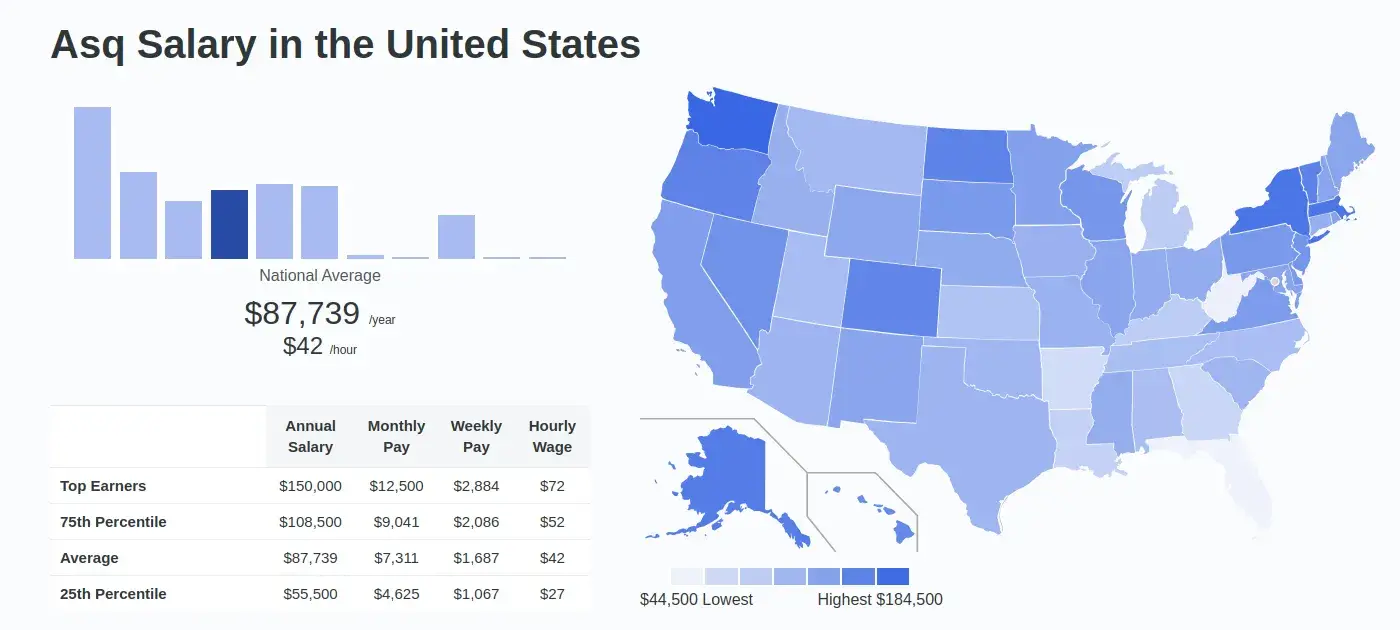
How Much Can You Make with the Certification?
Certified professionals can earn up to 40-percent more than their non-certified peers.
Certified Pharmaceutical GMP Professional Dumps FAQs
1.Are the exam screenshots provided by SPOTO real?
Yes every screenshot in our ASQ CPGP package is taken from actual candidate experiences. You'll see the same question styles, figures, and document excerpts that appear on the live exam.
2.Can I avail special discounts?
SPOTO regularly offers promotional pricing and bulk-purchase discounts. Contact our sales team to learn about current offers and volume packages.
3.How long is the service period of the ASQ CPGP dumps?
Your initial access period is 7-10 days ample time to complete multiple full-length practice exams and targeted drills. Extensions and renewals are available if you need additional preparation time.
4.How many questions are there in the ASQ CPGP exam dumps?
To ensure exhaustive coverage, we include 2-3× more questions than the actual 165-item exam. This extra breadth guarantees that you've rehearsed every topic and scenario before test day.